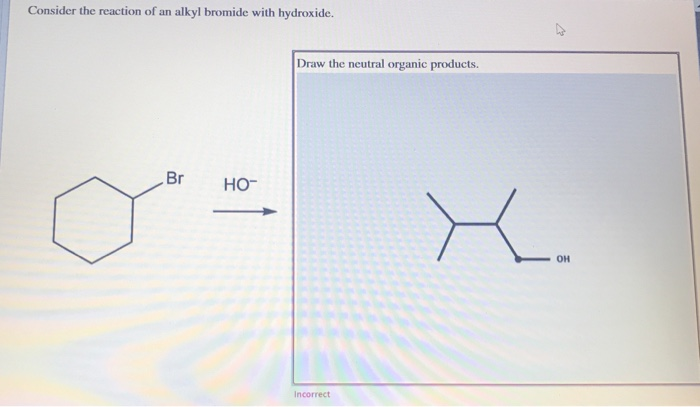
Consider the Reaction of an Alkyl Bromide With Hydroxide.
This problem has been solved. When comparing the relative rates in Table 72 which compares the relative rates of reaction for a series of alkyl bromides the following tend emerge.
Solved Consider The Reaction Of An Alkyl Bromide With Chegg Com
Draw the Sy2 product.
. D No reaction would take place. Draw the Sn2 product. True TF In order for ethers to undergo these reactions the leaving group must be an alkoxide and alkoxides are poor leaving groups.
66 a We know that when a secondary alkyl halide reacts with hydroxide ion by substitution the reaction occurs with inversion of configuration because the reaction is S N2. B The reaction would take place only with retention of configuration at the stereogenic center. Draw the SN2 product.
Consider the SN2 reaction between methyl bromide and the hydroxide ion. Of the starting alkyl halide Alkyl groups branching from the a and b carbons hinder the backside attack of the nucleophile resulting in a slower rate of reaction. Consider the dehydration of an.
Draw the major neutral organic compound recovered from each attempted. Draw the Sy2 product. Draw the Syl product.
Well talk this reaction through with a primary halogenoalkane to start with taking bromoethane as typical. A the reaction proceeds by the SN1 mechanism. Consider the reaction of an alkyl bromide with hydroxide.
The reaction proceeds by the Syl mechanism. -ОН Br Draw the Snl product. C2H5Bralc OH alc C2H5OHl Bralc The reaction is first order each in ethyl bromide and hydroxide ion.
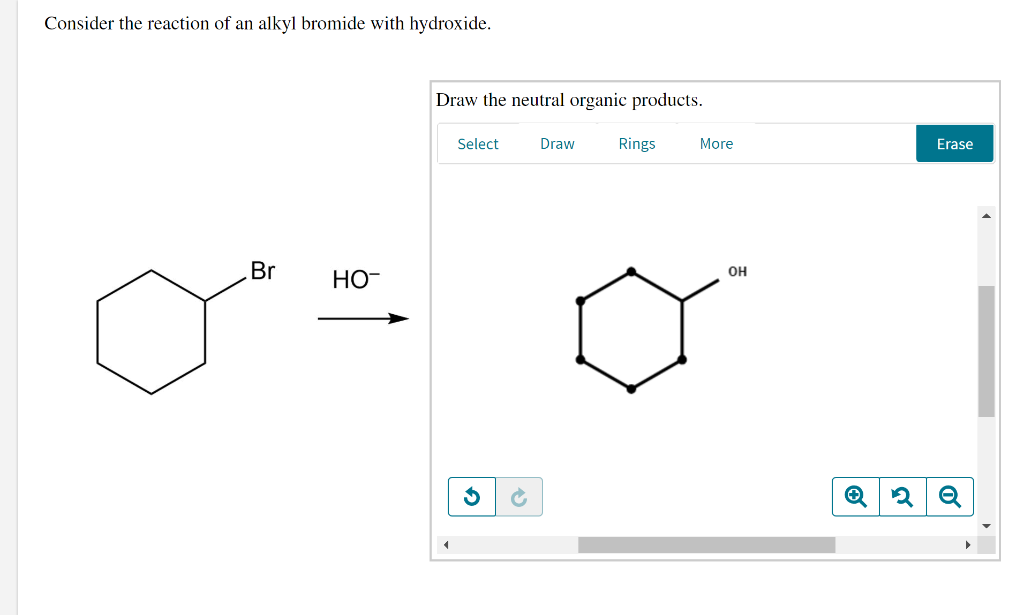
If we know that the configuration of -2-butanol from Section 58C is that shown here then we can conclude that -2-chlorobutane has the opposite configuration. Draw the neutral organic products. The reaction proceeds by the Sn1 mechanism.
Solution for Consider the reaction of an alkyl bromide and hydroxide ion. C The reaction would take place with racemization. The reaction of 1-bromobutane with sodium hydroxide affords the substitution product 1-butanol.
Consider the reaction of an alkyl bromide and hydroxide ion. Reaction of a primary alkyl halide with hydroxide will result in the formation of a primary _____. 86 7 ratings Transcribed image text.
Draw the SN2 product. Solution for Consider the reaction of an alkyl bromide and hydroxide ion. Draw the Sn2 product.
Rings Erase Select Draw Rings More. Select Draw More Erase Select Draw Rings Rings Get the answer to your homework problem. To get a clearer picture of the interplay of these factors consider the reaction of a 2º-alkyl halide isopropyl bromide with two different nucleophiles.
The nucleophilic substitution reaction - an SN2 reaction. Explain why a much better yield of primary amine is obtained from the reaction of an alkyl halide with azide ion N3. B The reaction exhibits a one-step mechanism.
Who are the experts. How will the rate of the reaction between bromomethane and hydroxide ion be affected if the following changes in concentration are made. The concentration of the alkyl halide is not changed and the concentration of the nucleophile is tripled.
E The alkyl halide does not possess a stereogenic center. The hydroxide anion is a poor _____ because it is a strong _____. Consider the substitution reaction that takes place when R-3-bromo-3-methylhexane is.
A Br This is a 2 carbocation step 2. A researcher attempts a substitution reaction with two different reagents water or hydroxide ion to see which route is better. In both cases there are two different sets of beta-hydrogens available to the elimination.
SN reaction OH OH Mechanism of SN reaction It Follows the steps below. The reaction proceeds by the SN1SN1 mechanism. Select Draw Rings More Erase.
Br- OH Draw the SyI product. Draw the major neutral organic product obtained if. What would be the effect on the reaction rate if the concentration of the alkyl halide and the concentration of the hydroxide ion are both doubled.
Br ГОН Draw the major neutral organic product obtained if. The reaction is fastest with primary alkyl halide. Draw the SN1 product.
When C2H5Br is 00478 M and OH is 0162 M the rate of disappearance of ethyl bromide is 28 10-7 Ms. The reaction between ethyl bromide C2H5Br and hydroxide ion in ethyl alcohol is shown below at 330 K. Br OH Draw the Si 1 product.
Select Draw Rings More Erase Select Draw Rings More Erase C H O C H O C 2 C MacBook Pro. Consider the reaction of hydroxide ion with methyl iodide to yield methanol. Ch06 Alkyl Halides landscapedocx Page 9.
Experts are tested by Chegg as specialists in their subject area. If we know that the configuration of -2-butanol from Section 58C is that shown here then we can conclude that -2-chlorobutane has the opposite configuration. The hydroxide ion is a good nucleophile since the oxygen atom has a negative charge and.
We review their content and use your feedback to keep the quality high. Consider the reaction of an alkyl bromide and hydroxide ion. The bromoethane has a polar bond between the carbon and.
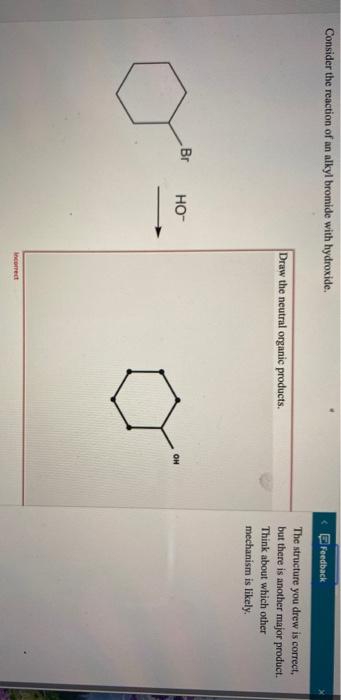
66 a We know that when a secondary alkyl halide reacts with hydroxide ion by substitution the reaction occurs with inversion of configuration because the reaction is S N2. Br OH Draw the SN1 product. Consider the reaction of an alkyl bromide and hydroxide ion.
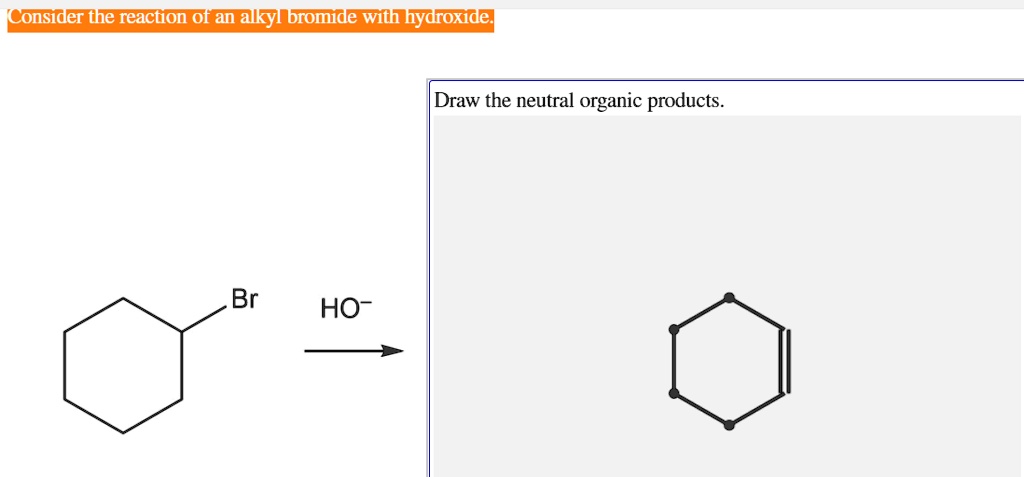
The leaving group detached from the compound. Consider the reaction of an alkyl bromide and hydroxide ion. By using the strongly basic hydroxide nucleophile we direct these reactions toward elimination.
What would happen to the rate of the reaction if the concentration of both 1-bromobutane and sodium hydroxide were doubled. In the case of the hydroxide ion there is a full negative charge on the oxygen as well as three lone pairs of electrons. The reaction proceeds by the SN2SN2 mechanism.
B the reaction proceeds by the SN2 mechanism. Br -OH Draw the major neutral organic product obtained if. Rearrangement of carbocation 3 carbocation step 3 Attack of The nucleophile - OH HO- product formed.
The answer is shown in the paper work attached. The reaction proceeds by the SN2 mechanism. Reaction conditions and the alkyl halide used.
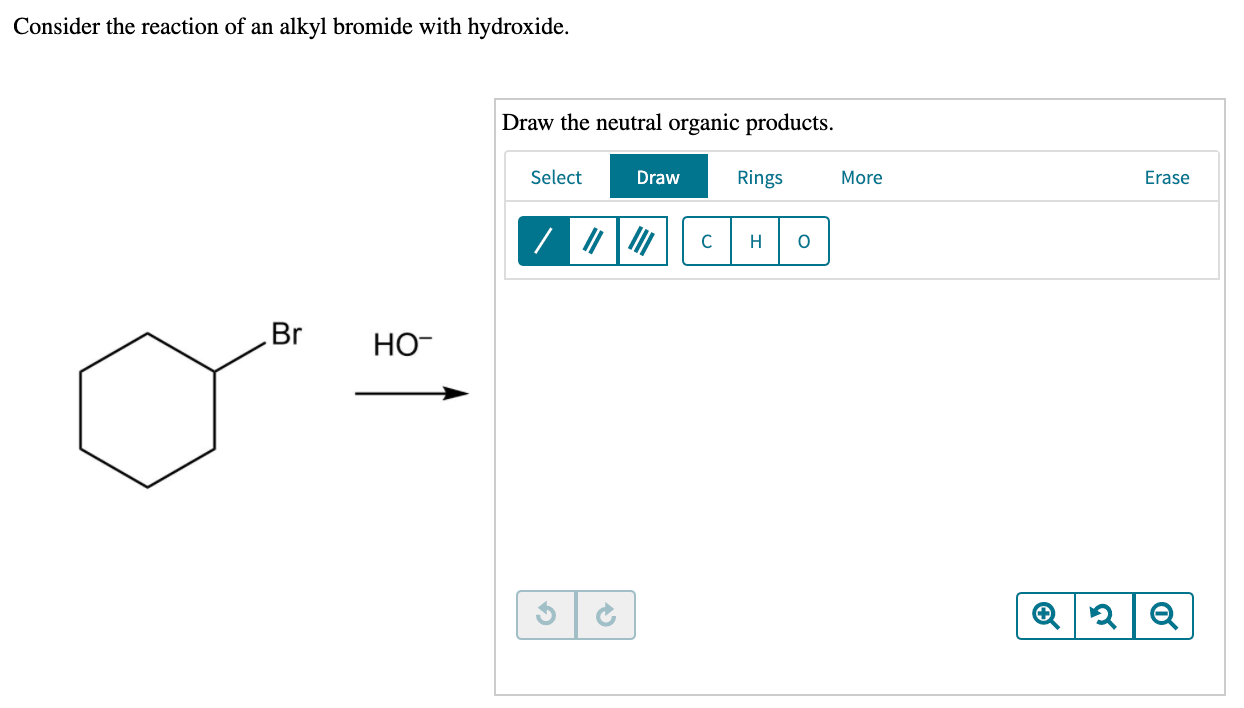
Draw the major neutral organic product obtained if. Consider the reaction of an alkyl bromide and hydroxide ion. Consider the reaction of an alkyl bromide with hydroxide Draw the neutral organic products 13.
Consider the reaction of an alkyl bromide and hydroxide ion. Select Draw Rings More Erase Select Draw Rings More Erase C 0. The concentration of the alkyl halide is cut in half and the concentration of the.
The reaction proceeds by the Sy2 mechanism. C The reaction rate.
Solved Consider The Reaction Of An Alkyl Bromide With Hydroxide Draw The Neutral Organic Products Ho
Solved Consider The Reaction Of An Alkyl Bromide With Chegg Com

Solved Consider The Reaction Of An Alkyl Bromide With Chegg Com
Solved Consider The Reaction Of An Alkyl Bromide With Chegg Com
No comments for "Consider the Reaction of an Alkyl Bromide With Hydroxide."
Post a Comment